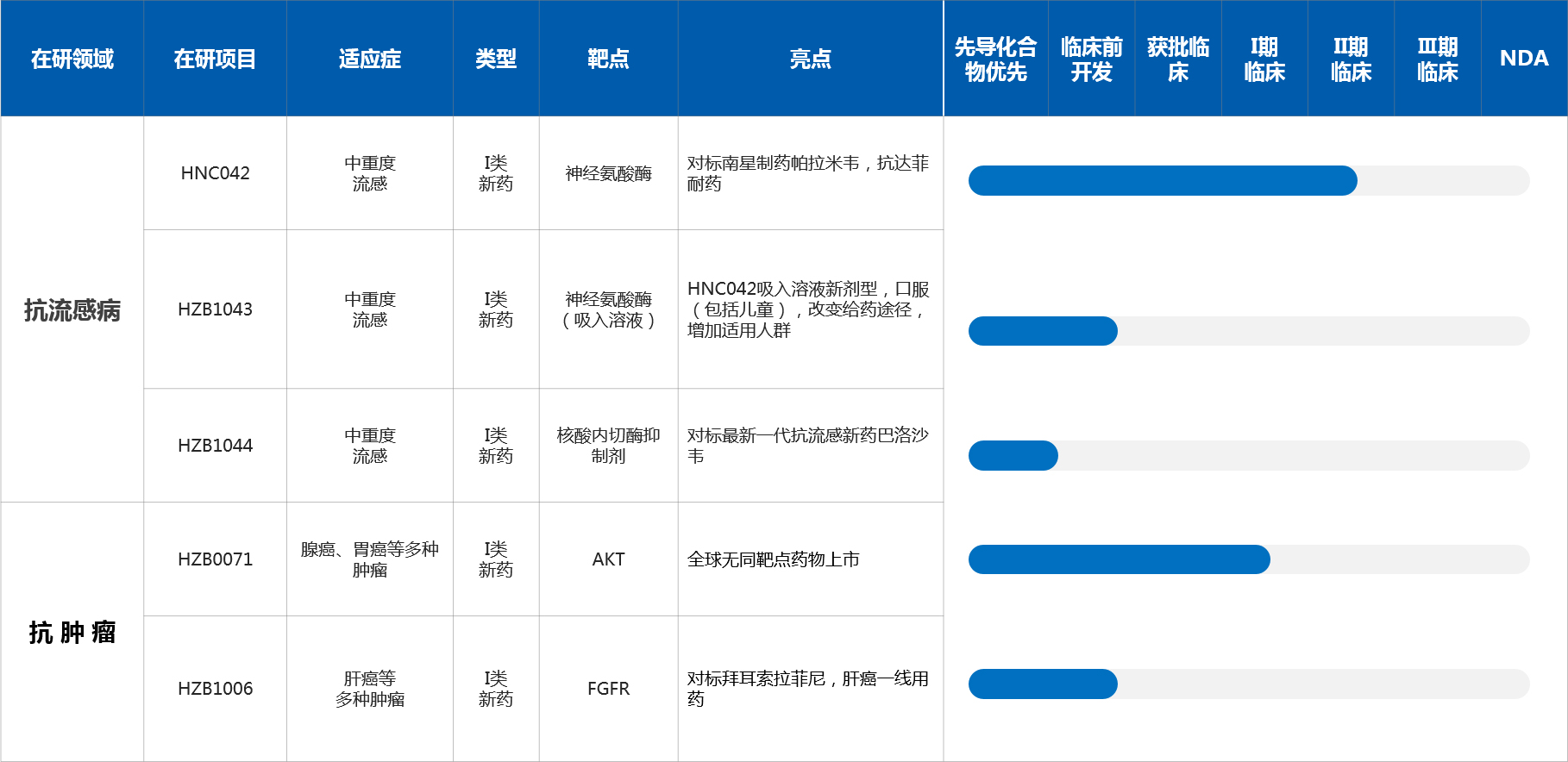
Drug Candidates
Chemical drugs: developing new drugs
HZB1043: Indications for moderate and severe influenza, class I drug, target neuraminidase (inhaled solution)
HZB1044: Indications for moderate and severe influenza, class I new drug, targeting endonuclease inhibitors
HZB1006: Indications for various tumors such as liver cancer, class I drug targets FGFR
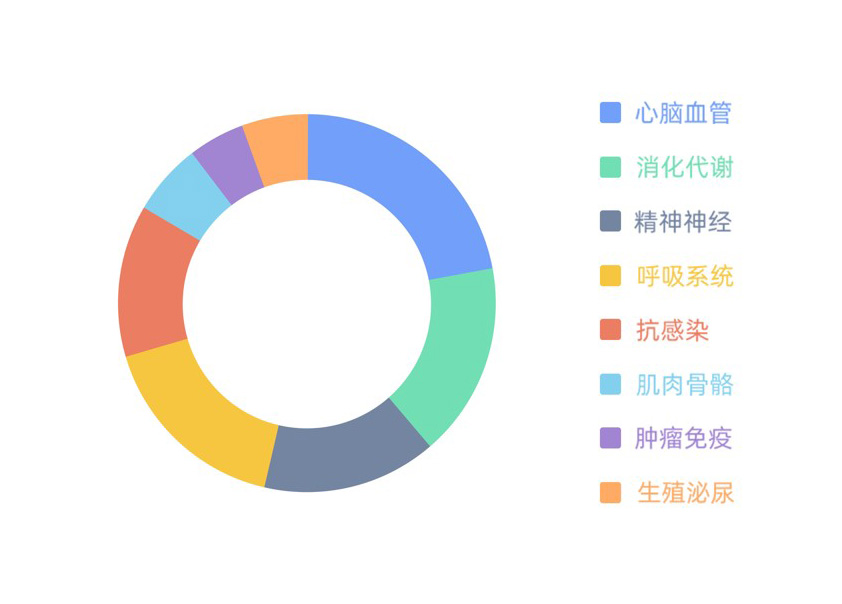
Generic drugs under research
There are a total of 68 generic projects under development, of which 14 are APIs and 54 FDFs. The current projects cover eight key therapies, CVS, digestion and metabolism, CNS, and respiratory systems as the core areas; infectious diseases, musculoskeletal system, tumor immunity, and genitourinary as the core intentions. Among these projects, 2 are in the process of issuing supplements, 2 are under BE studies, others will complete registration within 3 years and commercialied.
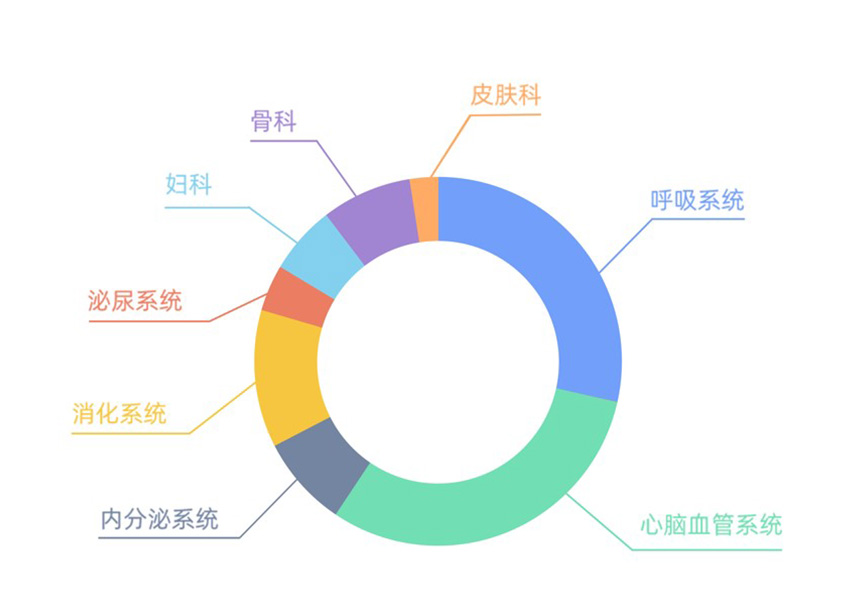
Hospital Drugs
Co-developed 49 hospital drugs
Granual Formulation:
559 products filled in Heilongjiang Province, 410 products filled in Anhui Province.
Xueshuantong Capsule
Upgrade the national standard, enters the 2020 edition of China Pharmacopoeia.
Pharmacodynamic research for Antibody influenza virus
Studies on Cytopathogenic inhibitory effects of virious Virus & inhibitory effects of Multi-drug resistant bacteria.
Xuesaitong injection
The research results have been applied to product production process quality control and project research standardization.
Acanthopanax injection
The standardized analysis was completed in cooperation with the Institute of Clinical Basic Medicine of Traditional Chinese Medicine, China Academy of Chinese Medical Sciences.
Children's hot clear syrup
14 clinical centers in China are conducting clinical trials and the results show favourable security.